熟肉制品中杂环胺的形成与抑制研究进展
张玉霞,周亚军*,李圣桡
(吉林大学食品科学与工程学院,吉林 长春 130062)
摘 要:杂环胺是熟肉制品中最常见的危害物,其种类繁多,形成途径复杂,抑制机制也不尽相同。本文介绍了2 类杂环胺的结构,主要阐述喹啉和喹喔啉类、2-氨基-1-甲基-6-苯基咪唑[4,5-b]吡啶、β-咔啉类(1-甲基-9H-吡啶[4,3-b]吲哚(1-methyl-9H-pyrido[4,3-b]indole,harman)、9H-吡啶[4,3-b]吲哚(9H-pyrido[4,3-b]indole,norharman))以及结合态杂环胺的形成途径,归纳减少杂环胺形成的措施及机理(控制加工条件和前体物、添加外源物质清除自由基和中间体、抑制美拉德反应等),并对目前的研究现状进行概括。
关键词:杂环胺;熟肉制品;形成;抑制;机理
杂环胺(heterocyclic aromatic amines,HAAs)是富含蛋白质的食品在热加工过程中产生的,是熟肉制品中常见的致癌物和致突变物。已有研究证实,在腌腊肉制品、酱卤肉制品、熏烧焙烤肉制品、干肉制品、油炸肉制品、肠类肉制品及火腿肉制品等34 种肉制品中均有杂环胺检出,并且9H-吡啶[4,3-b]吲哚(9H-pyrido[4,3-b]indole,norharman)和1-甲基-9H-吡啶[4,3-b]吲哚(1-methyl-9H-pyrido[4,3-b]indole,harman)在所有样品中均检测到[1]。杂环胺的致突变能力极强,是亚硝酸盐、黄曲霉毒素B1和苯并芘的数十倍甚至数百倍以上[2]。2-氨基-3-甲基咪唑[4,5-f]喹啉(2-amino-3-methylimidazo[4,5-f]quinoline,IQ)、2-氨基-3,4-二甲基咪唑[4,5-f]喹啉(2-amino-3,4-dimethylimidazo[4,5-ƒ]quinoline,MeIQ)、2-氨基-3,8-二甲基咪唑[4,5-f]喹喔啉(2-amino-3,8-dimethylimidazo[4,5-ƒ]quinoxaline,8-MeIQx)、2-氨基-1-甲基-6-苯基咪唑[4,5-b]吡啶(2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine,PhIP)、2-氨基-9H-吡啶[2,3-b]吲哚(2-amino-9H-pyrido[2,3-b]indol,AαC)、2-氨基-3-甲基-9H-吡啶[2,3-b]吲哚(2-amino-3-methyl-9H-pyrido[2,3-b]indol,MeAαC)、3-氨基-1,4-二甲基-5H-吡啶[4,3-b]吲哚(3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole,Trp-P-1)、3-氨基-1-甲基-5H-吡啶[4,3-b]吲哚(3-amino-1-methyl-5H-pyrido[4,3-b]indole,Trp-P-2)和2-氨基-6-甲基二吡啶[1,2-a:3’2’-d]咪唑(2-amino-6-methyldipyrido[1,2-a:3’2’-d]imidazole,Glu-P-1)都是极可疑或潜在的致癌物[3]。Harman和Norharman虽然本身不具有直接致癌性,但作为辅助突变物或潜在诱变剂,它们的存在会增强其他杂环胺的基因毒性[4-5]。流行病学研究表明,杂环胺会增加直肠癌、食道癌、帕金森症等疾病的患病风险[6]。熟肉制品在人们的膳食结构中所占的比例越来越大,科学家对其安全性的关注也持续增加。杂环胺作为熟肉制品中的主要危害物,探究杂环胺的形成和抑制机制已经成为研究人员广为关注的热点问题。
本文主要综述杂环胺的形成及抑制机制,尤其是熟肉制品中常见的几种杂环胺,简要概括目前杂环胺研究存在的问题,为后续研究和应用提供理论依据。
1 杂环胺的分类及结构
杂环胺属于多环芳烃类化合物,在100~300 ℃温度范围内形成的杂环胺称为热型杂环胺,又称IQ型杂环胺或氨基咪唑氮杂环芳烃(aminoimidazole-azaarenes,AIAs)类杂环胺;温度在300 ℃以上时,由蛋白质和单个氨基酸发生热解形成的杂环胺称为热解型杂环胺,又称非IQ型杂环胺或氨基咔啉类(amino-carbolines)杂环胺[7-9]。根据化学性质的不同,杂环胺又可以分为极性杂环胺和非极性杂环胺。迄今为止,人们已经从不同的食品体系中分离鉴定出超过30 种杂环胺[10],杂环胺的详细分类及结构如表1所示[7,9]。
表1 杂环胺的分类和结构
Table 1 Classif i cation and structures of heterocyclic aromatic amines
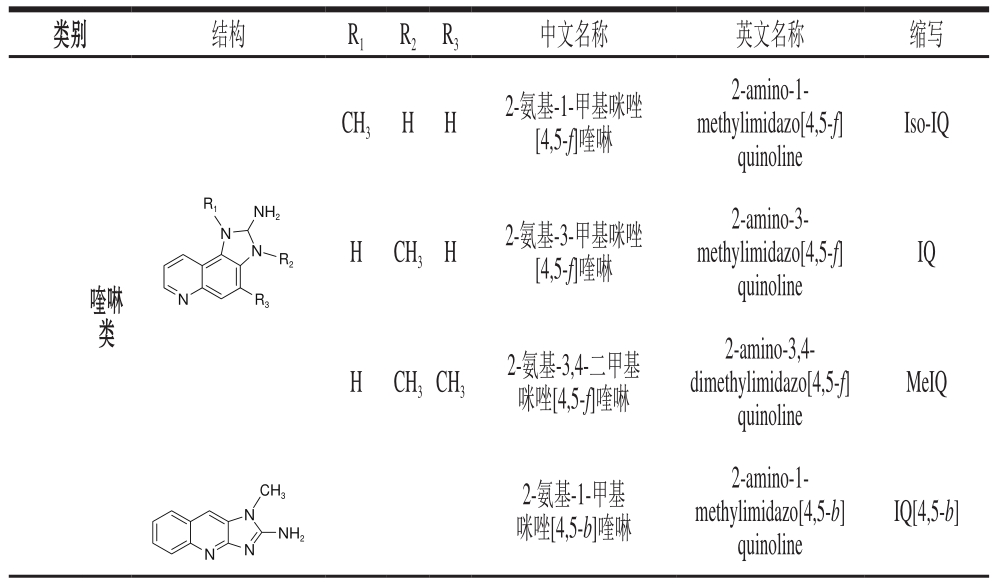
类别 结构 R1 R2 R3 中文名称 英文名称 缩写CH3 H H 2-氨基-1-甲基咪唑[4,5-f]喹啉2-amino-1-methylimidazo[4,5-f]quinoline Iso-IQ R1 NH2 N N R2 H CH3H 2-氨基-3-甲基咪唑[4,5-f]喹啉IQ喹啉类N R3 2-amino-3-methylimidazo[4,5-f]quinoline H CH3CH3 2-氨基-3,4-二甲基咪唑[4,5-f]喹啉2-amino-3,4-dimethylimidazo[4,5-f]quinoline MeIQ CH3 N IQ[4,5-b]NH2 2-氨基-1-甲基咪唑[4,5-b]喹啉N N 2-amino-1-methylimidazo[4,5-b]quinoline
续表1
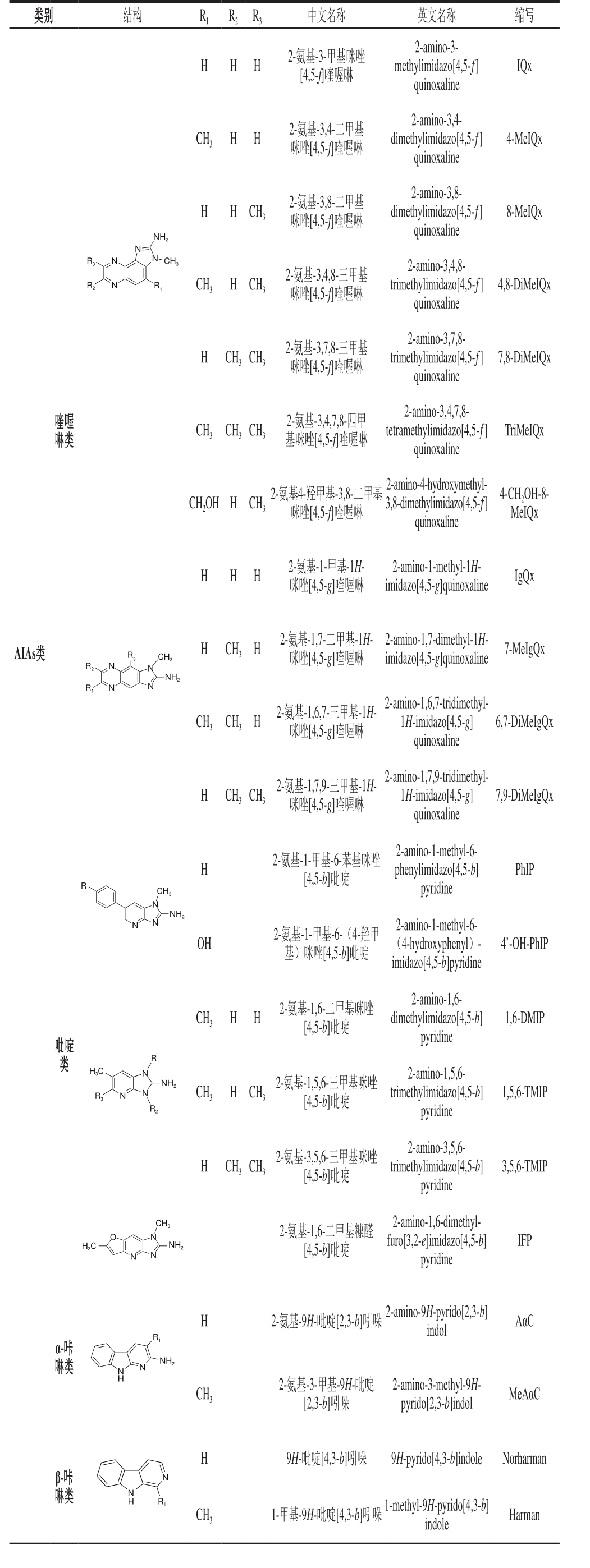
类别 结构 R1 R2 R3 中文名称 英文名称 缩写H H H 2-氨基-3-甲基咪唑[4,5-f]喹喔啉2-amino-3-methylimidazo[4,5-ƒ]quinoxaline IQx CH3 H H 2-氨基-3,4-二甲基咪唑[4,5-f]喹喔啉2-amino-3,4-dimethylimidazo[4,5-ƒ]quinoxaline 4-MeIQx H H CH3 2-氨基-3,8-二甲基咪唑[4,5-f]喹喔啉8-MeIQx NH2 2-amino-3,8-dimethylimidazo[4,5-ƒ]quinoxaline N R3N N CH3 R2 R1 N CH3 H CH3 2-氨基-3,4,8-三甲基咪唑[4,5-f]喹喔啉2-amino-3,4,8-trimethylimidazo[4,5-ƒ]quinoxaline 4,8-DiMeIQx H CH3CH3 2-氨基-3,7,8-三甲基咪唑[4,5-f]喹喔啉2-amino-3,7,8-trimethylimidazo[4,5-ƒ]quinoxaline 7,8-DiMeIQx喹喔啉类CH3 CH3CH3 2-氨基-3,4,7,8-四甲基咪唑[4,5-f]喹喔啉2-amino-3,4,7,8-tetramethylimidazo[4,5-ƒ]quinoxaline TriMeIQx CH2OH H CH3 2-氨基4-羟甲基-3,8-二甲基咪唑[4,5-f]喹喔啉2-amino-4-hydroxymethyl-3,8-dimethylimidazo[4,5-ƒ]quinoxaline 4-CH2OH-8-MeIQx H H H 2-氨基-1-甲基-1H-咪唑[4,5-g]喹喔啉2-amino-1-methyl-1H-imidazo[4,5-g]quinoxaline IgQx AIAs类R3 R2N N N CH3 NH2 H CH3H 2-氨基-1,7-二甲基-1H-咪唑[4,5-g]喹喔啉2-amino-1,7-dimethyl-1H-imidazo[4,5-g]quinoxaline 7-MeIgQx R1N CH3CH3H 2-氨基-1,6,7-三甲基-1H-咪唑[4,5-g]喹喔啉2-amino-1,6,7-tridimethyl-1H-imidazo[4,5-g]quinoxaline 6,7-DiMeIgQx H CH3CH3 2-氨基-1,7,9-三甲基-1H-咪唑[4,5-g]喹喔啉2-amino-1,7,9-tridimethyl-1H-imidazo[4,5-g]quinoxaline 7,9-DiMeIgQx H PhIP R1 CH3 2-氨基-1-甲基-6-苯基咪唑[4,5-b]吡啶2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine N N N NH2 OH 2-氨基-1-甲基-6-(4-羟甲基)咪唑[4,5-b]吡啶2-amino-1-methyl-6-(4-hydroxyphenyl)-imidazo[4,5-b]pyridine 4’-OH-PhIP CH3 H H 2-氨基-1,6-二甲基咪唑[4,5-b]吡啶1,6-DMIP吡啶类2-amino-1,6-dimethylimidazo[4,5-b]pyridine R1 H3C R3 N NH2 N N CH3 H CH3 1,5,6-TMIP R2 2-氨基-1,5,6-三甲基咪唑[4,5-b]吡啶2-amino-1,5,6-trimethylimidazo[4,5-b]pyridine H CH3CH3 2-氨基-3,5,6-三甲基咪唑[4,5-b]吡啶2-amino-3,5,6-trimethylimidazo[4,5-b]pyridine 3,5,6-TMIP O N H3C CH3 NH2 IFP N N 2-氨基-1,6-二甲基糠醛[4,5-b]吡啶2-amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine H R1 2-氨基-9H-吡啶[2,3-b]吲哚 2-amino-9H-pyrido[2,3-b]indol AαC α-咔啉类 NH2 N NH CH3 2-氨基-3-甲基-9H-吡啶[2,3-b]吲哚2-amino-3-methyl-9H-pyrido[2,3-b]indol MeAαC H 9H-吡啶[4,3-b]吲哚 9H-pyrido[4,3-b]indole Norharman β-咔啉类N N H R1 CH3 1-甲基-9H-吡啶[4,3-b]吲哚 1-methyl-9H-pyrido[4,3-b]indole Harman
续表1
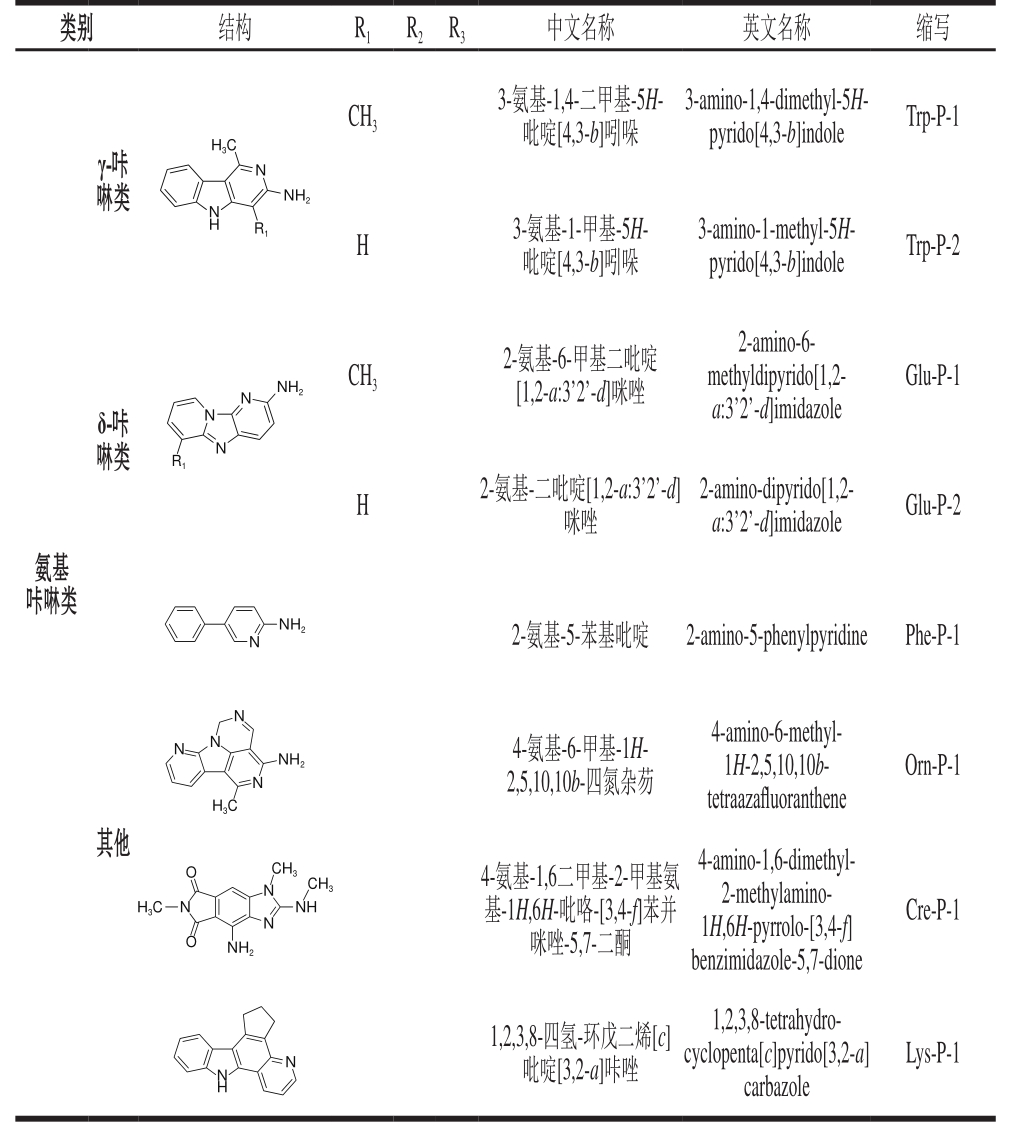
类别 结构 R1 R2 R3 中文名称 英文名称 缩写CH3 H3C 3-氨基-1,4-二甲基-5H-吡啶[4,3-b]吲哚3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole Trp-P-1 γ-咔啉类 NH2 N N H R1 H 3-氨基-1-甲基-5H-吡啶[4,3-b]吲哚3-amino-1-methyl-5H-pyrido[4,3-b]indole Trp-P-2 Glu-P-1 N NH2 CH3 2-氨基-6-甲基二吡啶[1,2-a:3’2’-d]咪唑δ-咔啉类N 2-amino-6-methyldipyrido[1,2-a:3’2’-d]imidazole R1 N H 2-氨基-二吡啶[1,2-a:3’2’-d]咪唑2-amino-dipyrido[1,2-a:3’2’-d]imidazole Glu-P-2氨基咔啉类NH2N 2-氨基-5-苯基吡啶 2-amino-5-phenylpyridine Phe-P-1 N N N NH2 N 4-氨基-6-甲基-1H-2,5,10,10b-四氮杂芴Orn-P-1 H3C 4-amino-6-methyl-1H-2,5,10,10btetraazaf l uoranthene其他CH3 H3C O CH3 N O N N NH Cre-P-1 NH2 4-氨基-1,6二甲基-2-甲基氨基-1H,6H-吡咯-[3,4-f]苯并咪唑-5,7-二酮4-amino-1,6-dimethyl-2-methylamino-1H,6H-pyrrolo-[3,4-f]benzimidazole-5,7-dione Lys-P-1 NH N 1,2,3,8-四氢-环戊二烯[c]吡啶[3,2-a]咔唑1,2,3,8-tetrahydrocyclopenta[c]pyrido[3,2-a]carbazole
2 杂环胺的形成机理
杂环胺是通过复杂的美拉德反应生成的,主要前体物质有肌酸(肌酸酐)、氨基酸、糖类及一些二肽,这些前体物质均广泛存在于肌肉组织中[11]。
2.1 AIAs类杂环胺的形成
目前,AIAs类杂环胺的形成机制研究较多也较为深入。AIAs类杂环胺均含有1 个N-甲基-氨基咪唑主体,这类杂环胺可以先由糖类和氨基酸经过脱水、环化形成吡啶或吡嗪,肌酸在温度高于100 ℃时通过自发的环化和脱水形成2-氨基咪唑部分,二者再与Strecker降解产生的醛类发生醇醛缩合,形成相应的咪唑喹啉、咪唑喹喔啉和咪唑吡啶类化合物[11-13]。该途径已经在MeIQx、4,8-DiMeIQx和7,8-DiMeIQx的合成和鉴定中得到验证。另有研究表明,肌酸也可以先直接与醛类发生缩合反应[14]。除此之外,AIAs类杂环胺还可以通过自由基形成。该途径中烷基吡啶自由基和二烷基吡嗪自由基分别与肌酸酐反应,形成喹啉和喹喔啉类杂环胺。1992年,Pearson等[15]提出,IQ和MeIQx可以通过烷基吡啶自由基和肌酸酐反应生成,而二烷基吡嗪自由基和肌酸酐反应时则会生成MeIQx和DiMeIQx。此后,Kato[16]、Kikugawa[17]等在Pearson的研究基础上进一步探索,分别于1996年和1999年进一步证实和完善了自由基形成杂环胺的途径,得到目前普遍被研究人员认可的杂环胺生成的自由基机制:氨基酸与糖类在美拉德反应的初级阶段,没有发生Amadori重排前,先形成吡啶自由基、吡嗪自由基和碳中心自由基,由于所形成的自由基非常不稳定,在体系内会进一步形成相应的吡啶和吡嗪衍生物,最后再与肌酸酐反应形成AIAs类杂环胺。具体形成途径如图1所示。
PhIP是熟肉制品中最为常见也是含量较多的杂环胺之一,有关PhIP的研究报道较其他杂环胺来说相对更多,其形成机理的研究也更为深入。苯丙氨酸和肌酸酐(肌酸)是PhIP形成的重要前体物质的结论已经被认同和证实[18],苯乙醛则是PhIP形成的重要中间产物[19-20]。通过13C标记法也已表明,PhIP中的吡啶部分来自于苯丙氨酸的碳原子,而PhIP的咪唑部分则由肌酸酐形成。除此之外,苯丙氨酸中的氨基氮和苯环以及肌酸中的氨基氮和环内1位的氮原子也参与PhIP的形成[21]。PhIP的形成途径如图1B所示:首先,苯丙氨酸经过Strecker降解生成苯乙醛;接着苯乙醛与肌酸酐发生醇醛缩合反应形成羟醛加合物,这种加合物极不稳定,会继续脱水形成相应的羟醛缩合物;最后,羟醛缩合物和苯乙醛反应产生甲醛,肌酸酐降解过程中生成氨,甲醛和氨进一步发生反应,最终形成PhIP[9,11,20-21]。也有研究表明,除苯丙氨酸外,其他氨基酸(如酪氨酸、亮氨酸、异亮氨酸等)和核酸与肌酸酐共同加热发生反应也可以生成PhIP[22-23];并且糖类虽然没有直接参与PhIP的生成反应,但却对PhIP的生成起着非常显著的作用,已证明葡萄糖、四碳糖(赤藓糖)、树胶醛糖和半乳糖均会影响PhIP的生成[24]。
2.2 氨基咔啉类杂环胺的形成
相对于AIAs类杂环胺,氨基咔啉类杂环胺的研究报道较少,其形成途径尚不明确,大多认为氨基咔啉类杂环胺形成的主要途径是蛋白质或氨基酸的高温热解[9,11]。
目前,β-咔啉类杂环胺中的Norharman的形成途径(图2)已经较为明确:色氨酸先经Amadori重排后脱水,形成的脱水产物在环氧孤对电子的作用下通过β-消去反应形成共轭的氧鎓离子;然后氧鎓离子通过脱水和形成共轭体系来保持自身稳定或经C-C键断开后进行分子内的亲核取代,进而形成β-咔啉类杂环胺[25]。和Norharman相同,Harman的前体物质也是色氨酸和葡萄糖。除上述途径外,也有报道表明,β-咔啉可以由色氨酸与乙醛或α-酮酸通过皮克泰-斯宾格勒反应形成四氢化-β-咔啉(tetrahydro-β-carbolines,THβC)后,再经进一步的氧化、脱羧等反应生成[26]。由此得到,THβC、1,2,3,4-四氢化-β-咔啉-3-羧酸(1,2,3,4-tetrahydroβ-carboline-3-carboxylic acid,THCA)和1-甲基-1,2,3,4-四氢化-β-咔啉-3-羧酸(1-methyl-1,2,3,4-tetrahydro-βcarbolie-3-carboxylic,MTCA)也是Norharman和Harman的中间体[9,27]。与其他热解型杂环胺相比,即使在不超过100 ℃时,延长加热时间也会产生大量的β-咔啉,中国传统酱卤肉制品以及香肠、火腿等在生产加工过程中产生的杂环胺主要为Harman和Norharman。另有研究发现,Trp-P-1和Trp-P-2可通过含有肌酸酐、葡萄糖、色氨酸或异亮氨酸的混合体系在180 ℃条件下加热产生[28];AαC和MeAαC可通过大豆球蛋白的热解产生[29];Glu-P-1和Glu-P-2则可经谷氨酸热解产生[30];但这些杂环胺的具体形成途径尚不明确。
氨基咔啉类杂环胺的生成途径复杂多变,即使没有肌酸(肌酸酐)的参与也可以产生,此类杂环胺是人们日常饮食中摄入最普遍的杂环胺。因此,很有必要对氨基咔啉类杂环胺的形成机制进行更为深入的研究。
2.3 结合态杂环胺的形成
熟肉制品中不只存在游离态杂环胺,还有一部分杂环胺以结合态形式存在。结合态杂环胺的形成方式有很多种:游离态杂环胺中的氨基与蛋白质或氨基酸中的羧基反应形成稳定的酰胺键,最终形成杂环胺-氨基酸加合物或杂环胺-蛋白质加合物;游离态杂环胺可以选择性吸附到某些蛋白质聚合物的表面,形成结合态杂环胺;蛋白质中的氨基酸残基与其他杂环胺前体(如葡萄糖、肌酸和肌酸酐)发生反应,形成结合态杂环胺[31-32]。李永[11]、Kataoka[33-34]等发现,PhIP、IQ、MeIQx和Trp-P-1可以与多种游离氨基酸的活性羟基反应,形成杂环胺-氨基酸加合物,甘氨酸(Gly)、脯氨酸和组氨酸与PhIP、IQ、MeIQx和Trp-P-1形成加合物较容易,而丝氨酸、半胱氨酸和赖氨酸则很难与杂环胺形成加合物。结合态杂环胺在体内消化酶等物质的作用下,存在重新转化为游离态的风险,因此结合态杂环胺也是评估杂环胺致癌风险的重要因素之一[11,31-33]。
3 杂环胺的抑制及其机制
熟肉制品是原料肉经过热加工处理制作而成,既含有杂环胺形成所需要的前体物质,又具备形成杂环胺的温度等条件,因此控制熟肉制品中杂环胺的生成量十分必要。
3.1 控制加工条件和加工方式
不同的加热时间、加热温度及加热方式对杂环胺生成的种类和数量影响较大。研究发现,加热温度越高,加热时间越长,杂环胺的种类和含量均会明显增加;但在湿热体系中加热时间过长时,杂环胺的含量反而会有所下降[7,35]。Knize等[36]发现,随着加热时间的不断延长,4,8-DiMeIQx的生成量最终会趋于稳定,8-MeIQx和7,8-DiMeIQx的生成量则呈现先升高后降低的趋势。由此可见,在长时间加热的条件下,杂环胺在生成的同时也可能发生降解。Arvidsson等[37]发现,PhIP最易发生降解,其次为7,8-DiMeIQx、8-MeIQx、4,8-DiMeIQx和IQx。除此之外,温和的加热方式,如蒸、煮等产生的杂环胺较少[38];用干热方式加热,如平底锅煎制等方式会产生较多的杂环胺[39];微波加热对杂环胺形成的抑制效果说法不一[40-42]。通过改变加热方式来抑制杂环胺的形成是基于传热方式和传热介质的不同,因此在实际操作中需要综合考虑加热时间、温度及加热方式的影响[39,43]。
3.2 控制前体物的种类及含量
糖类、氨基酸、肌酸(肌酸酐)等是杂环胺形成的重要前体物质,控制其种类和含量可以起到减少杂环胺生成的作用。糖类对杂环胺形成影响显著,研究发现,当糖类含量超过肌酸含量时,糖类对杂环胺的生成起到抑制作用[44],这可能是由于过量的糖类在美拉德反应中优先生成5-羟甲基-2-糠醛,通过与肌酸酐反应抑制了杂环胺的形成[45];糖的种类不同,其对杂环胺生成的影响也不同。Hasnol等[46]发现,富含葡萄糖和果糖的蜂蜜对杂环胺有很好的抑制效果,可以取代甜卤汁中的蔗糖。苯丙氨酸、色氨酸等游离氨基酸是形成PhIP、Harman和Norharman的重要前体物,降低原料中苯丙氨酸和色氨酸的含量可以起到抑制PhIP、Harman和Norharman形成的作用。控制前体物的比例也可以抑制杂环胺的形成,研究发现,肉中天然存在的糖类约占肌酸和氨基酸物质的量的一半;如果糖类的含量超过这个比例,杂环胺的形成量就会减少[47]。金属阳离子会影响PhIP形成过程中苯乙醛与肌酸酐的反应。Yu Shujuan等[48]发现,Fe2+和Fe3+可以抑制PhIP的形成,原因可能是Fe2+和Fe3+与肌酸酐中的氨基发生反应,形成新的化合物或Stresker醛,从而阻断苯乙醛与肌酸酐的反应。由此可见,减少肌酸酐含量可以抑制杂环胺的生成。
3.3 清除自由基
AIAs类杂环胺可以通过自由基途径生成,因此具有自由基清除作用的物质可以抑制杂环胺的形成。从结构上看,酚类物质苯环上羟基的活性氧起到抗氧化的作用,可以清除反应过程中生成的自由基,抑制杂环胺的形成[49]。
植物组织及果实中含有很多酚类化合物,葡萄籽中葡萄多酚含量较高,茶中茶多酚含量为20%~35%。许多香辛料和植物提取物中富含酚类物质,在熟肉制品加工过程中加入香辛料和植物提取物既可以抑制杂环胺的形成[11],同时又能起到调味和防腐的作用。食品中常见的酚类抗氧化物主要有3 类:黄酮、酚萜类化合物和儿茶素[50]。Sepahpour等[51]发现,姜黄、咖喱叶、火炬姜和柠檬草单一或混合加入均可降低腌制烤牛肉中的杂环胺总形成量。Shao Zeping等[52]研究发现,烷氧自由基是MeIQx和4,8-DiMeIQx形成过程中的重要物质,9 种黄酮类物质可以通过清除烷氧自由基来抑制杂环胺的形成,但具体抑制途径比单纯清除烷基自由基复杂。大量研究表明,葡萄籽提取物[53]、红辣椒提取物[54]、苹果皮提取物[55]、玫瑰茶提取物[56]、橄榄提取物[57]、甘蔗糖蜜提取物[58]、菊花提取物[59]及茶叶提取物[60]等对杂环胺的抑制作用与自由基清除能力有关。香辛料和植物提取物虽然对杂环胺总量有抑制作用,但对单一杂环胺的抑制效果并不相同:在抑制某种杂环胺的同时可能也会促进另外某种杂环胺的形成。香叶对酱牛肉中Harman和Norharman有明显抑制作用,却能促进MeIQ的形成;良姜可以抑制Harman和Norharman的形成,而槲皮素和山柰酚却对Harman和Norharman的形成有明显促进作用[61]。
此外,VC和异VC能清除吡嗪阳离子自由基,从而抑制杂环胺的生成[62]。半胱氨酸、乙酰半胱氨酸等含硫氨基酸可能通过清除美拉德反应过程中的吡嗪阳离子自由基来抑制杂环胺的生成[63],但具体机制仍需进一步验证。啤酒腌制可以减少炭烤猪肉中杂环胺的含量[64],朝鲜蓟提取物可以减少牛肉和鸡胸肉中杂环胺的含量[65],这与啤酒和朝鲜蓟提取物的抗氧化能力有关。
3.4 清除中间体
苯乙醛是PhIP形成过程中的重要中间体,黄酮类化合物可以与苯乙醛形成加合物,从而抑制PhIP的形成。秦川[66]比较8 种黄酮类化合物对烤肉中PhIP形成的抑制作用与2,2’-联氮双-(3-乙基苯并噻唑啉-6-磺酸)二铵盐自由基清除能力和苯乙醛清除能力的相关性,发现抑制作用与前者不存在显著的相关性,与后者呈正相关,在烤肉中共检测出5 种黄酮-苯乙醛加合物。李利洁[40]发现,向微波场下的牛肉中加入槲皮素,检测到槲皮素-苯乙醛加合物,其含量随着槲皮素含量的增大而增加,对PhIP形成的抑制作用也增加。二氢杨梅素[67]、VB1、VB3、VB6和VB7[68]可以捕获苯乙醛;柚皮素对苯乙醛、甲醛和乙醛具有清除作用[9];某些氨基酸的氨基可以与苯乙醛的羰基结合,起到抑制杂环胺形成的作用[69]。
酰胺类物质也可以清除中间体,抑制杂环胺的生成。辣椒、花椒、黑胡椒中的辣椒素、花椒麻素和胡椒碱都是酰胺类物质,研究表明,辣椒素和胡椒碱对PhIP的中间体苯乙醛有明显的抑制作用,胡椒碱对喹喔啉类杂环胺形成的中间体,即甲醛、乙醛和2,5-二甲基吡嗪有显著的抑制效果[70-71]。除了苯乙醛、甲醛和乙醛外,对于Harman和Norharman形成的中间体THβC、THCA和MTCA以及其他杂环胺形成的中间体的清除措施研究很少。
3.5 抑制美拉德反应
许多研究表明,杂环胺是通过复杂的美拉德反应形成的。含硫化合物可以抑制美拉德反应,从而降低杂环胺的生成量。亚硫酸钠、亚硫酸氢钠以及洋葱和大蒜中的有机硫化合物可以有效抑制杂环胺的形成[11,72-74]。Tsai等[75]将6 种有机硫化合物二烯丙基二硫化物(diallyl disulf i de,DAD)、二丙基二硫化物(dipropyl disulf i de,DPD)、二烯丙基硫醚、烯丙基甲基硫醚、烯丙基硫醇和半胱氨酸加入到猪肉汁中,发现其均可减少IQ、MeIQ和MeIQx的生成量。Shin等[76]提出,DAD、DPD和氨基酸均与葡萄糖发生反应,这种竞争性反应可以抑制美拉德反应,这可能是导致杂环胺生成量减少的原因。此外,含硫氨基酸可以有效抑制热解氨基酸葡萄糖体系下的非酶促褐变反应[77]。有研究表明,谷胱甘肽、L-半胱氨酸、L-胱氨酸和脱氧蒜氨酸等含硫氨基酸能显著降低模型体系和炸牛肉饼中杂环胺的含量[78]。
3.6 其他
控制食品中的其他成分也可以有效减少熟肉制品中杂环胺的形成。水分的存在会对杂环胺生成有一定的抑制作用。持水性物质会减少杂环胺的形成,例如,微晶纤维素和羧甲基纤维素有很好的持水性和保水性,用于烤牛肉饼中可以抑制杂环胺的形成[79];氯化钠和三聚磷酸盐等加入到熟肉制品中,可以显著降低PhIP的含量[80]。脂肪氧化产生自由基,会促进杂环胺的形成,而多不饱和脂肪酸具有一定的抗氧化能力,可以抵消因氧化产生自由基对杂环胺形成的促进作用,因此富含多不饱和脂肪酸的初榨橄榄油对杂环胺的形成有一定的抑制作用[81-82]。
4 结 语
杂环胺是熟肉制品中常见的危害物之一。杂环胺的种类较多,形成机理复杂,再加上食品体系自身的复杂性,大大增加了对杂环胺形成机理和抑制机制的研究难度。目前,研究人员仅对几种常见杂环胺的形成机理和抑制机制进行了探索。
首先,由于同种杂环胺的形成途径不一,仅PhIP、Norharman等少数几种杂环胺有较为明确的生成途径,但当前发现的形成假说也存在争议,仍需要后续进一步的探索研究。其次,在杂环胺的控制方面,目前的研究多停留在某种物质或方法可以产生抑制杂环胺的效果,但对于该物质和方法究竟通过哪种途径抑制杂环胺没有深入研究,例如,黄酮类物质在抑制杂环胺形成时,可能是由于既清除了自由基,又形成了黄酮-中间体加合物,今后需将研究重点放在抑制机制上。再者,当前的研究绝大多数针对游离态杂环胺,对结合态杂环胺的研究较少,而结合态杂环胺存在重新恢复游离态的风险,因此需要进一步探究结合态杂环胺在体外和体内的稳定性及抑制措施等。最后,由于食品体系过于复杂,许多研究仍然停留在模拟体系的基础上,在食品体系中是否有效还需进一步验证,而且尽管杂环胺具有致癌和致畸作用,但缺乏关于杂环胺在食品中含量标准合理建议的报道。总之,后续研究的重点应该放在拓宽杂环胺种类研究和深入研究食品体系中杂环胺的形成机理及抑制机制上,为熟肉制品中杂环胺的控制提供技术支持和理论依据。
参考文献:
[1] 潘晗. 酱肉中Norharman和Harman形成机理的研究[D]. 北京: 中国农业科学院, 2014: 11-19.
[2] 谢洋洋, 王小溪, 闫文杰, 等. 肉制品中杂环胺的研究进展[J].食品研究与开发, 2017, 38(15): 199-205. DOI:10.3969/j.issn.1005-6521.2017.15.041.
[3] International Agency for Research on Cancer. Some naturally occuring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins[M]//IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: IARC, 1993:245-395.
[4] ALVES R C, MENDES E, OLIVEIRA B P P, et a1. Norharman and harman in instant coffee and coffee substitutes[J]. Food Chemistry,2010, 120(4): 1238-1241. DOI:10.1016/j.foodchem.2009.11.070.
[5] BOEIRA M, VIANA F, PICADA N, et al. Genotoxic and recombinogenic activities of the two β-carboline alkaloids harman and harmine in saccharomyces[J]. Mutation Research/Fundamental and Molecular Mechanism of Mutagenesis, 2002, 500(1/2): 39-48.DOI:10.1016/S0027-5107(01)00294-9.
[6] CHENG Kawing, CHEN Feng, WANG Mingfu. Heterocyclic amines:chemistry and health[J]. Molecular Nutrition and Food Research,2006, 50(12): 1150-1170. DOI:10.1002/mnfr.200600086.
[7] 姚瑶, 彭增起, 邵斌, 等. 加工肉制品中杂环胺的研究进展[J]. 食品科学, 2010, 31(23): 447-453.
[8] JAGERSTAD M, SKOG K, ARVIDSSON P, et al. Chemistry,formation, and occurrence of genotoxic heterocyclic amines identif i ed in model systems and cooked foods[J]. European Food Research and Technology, 1998, 207(6): 419-427. DOI:10.1007/s002170050355.
[9] 薛超轶, 何志勇, 高大明, 等. 加工肉制品中杂环胺的研究进展[J].食品安全质量检测学报, 2018, 9(14): 3590-3597.
[10] GIBIS M. Heterocyclic aromatic amines in cooked meat products:causes, formation, occurrence, and risk assessment[J]. Comprehensive Reviews in Food Science and Food Safety, 2016, 15(2): 269-302.DOI:10.1111/1541-4337.12186.
[11] 李永, 何志勇, 高大明, 等. 热加工食品中杂环胺形成及抑制机制[J].食品安全质量检测学报, 2019, 10(2): 312-319.
[12] 鄢嫣. 烤肉中杂环胺的形成规律的研究[D]. 无锡: 江南大学, 2015:2-4; 97-105.
[13] WONG Daniel, CHENG Kawing, WANG Mingfu. Inhibition of heterocyclic amine formation by water-soluble vitamins in maillard reaction model systems and beef patties[J]. Food Chemistry, 2012,133(3): 760-766. DOI:10.1016/j.foodchem.2012.01.089.
[14] BORDAS M, MOYANO E, PUIGNOU L, et al. Formation and stabiltemperature, time and precursors[J]. Journal of Chromatography B,2004, 802(1): 11-17. DOI:10.1016/j.jchromb.2003.09.024.
[15] PEARSON A M, CHEN C, GRAY J I, et al. Mechanism(s)involved in meat mutagen formation and inhibition[J]. Free Radical Biology and Medicine, 1992, 13(2): 161-167. DOI:10.1016/0891-5849(92)90078-U.
[16] KATO K, HARASHIMA T, MORIYA N, et a1. Formation of the mutagenic/carcinogenic imidazoquinoxaline-type heterocyclic amines through the unstable free radical Maillard intermediates and its inhibition by phenolic antioxidants[J]. Carcinogenesis, 1996, 17(11):2469-2476. DOI:10.1093/carcin/17.11.2469.
[17] KIKUGAWA K. Involvement of free radicals in the formation of heterocyclic amines and prevention by antioxidants[J]. Cancer Letters,1999, 143(2): 123-126. DOI:10.1016/S0304-3835(99)00140-8.
[18] JINAP S, MOHD-MOKHTAR M S, FARHADIAN A, et al. Effects of varying degrees of doneness on the formation of heterocyclic aromatic amines in chicken and beef satay[J]. Meat Science, 2013(2): 202-220.DOI:10.1016/j.meatsci.2013.01.013.
[19] ZÖCHLING S, MURKOVIC M. Formation of the heterocyclic aromatic amine phip: identif i cation of precursors and intermediates[J].Food Chemistry, 2002, 79(1): 125-134. DOI:10.1016/S0308-8146(02)00214-5.
[20] CHENG K W, WONG C C, CHO C K, et al. Trapping of phenylacetaldehyde as a key mechanism responsible for naringenin’s inhibitory activity in mutagenic 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine formation[J]. Chemical Research in Toxicology, 2008, 21(10): 2026-2034. DOI:10.1021/tx800220h.
[21] ZAMORA R, ALCÓN E, HIDALGO F J. Ammonia and formaldehyde participate in the formation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in addition to create(ni)ne and phenylacetaldehyde[J]. Food Chemistry, 2014, 155: 74-80.DOI:10.1016/j.foodchem.2014.01.039.
[22] SKOG K. Cooking procedures and food mutagens: a literature review[J]. Food and Chemical Toxicology, 1993, 31(9): 655-675.DOI:10.1016/0278-6915(93)90049-5.
[23] MANABE S, KURIHARA N, SHIBUTANI T, et al. Nucleicacids induce the formation of a carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in a model system[J].Carcinogenesis, 1993, 14(5): 903-906. DOI:10.1093/carcin/14.5.903.
[24] SKOG K, JÄGERSTAD M. Effects of glucose on the formation of PhIP in a model system[J]. Carcinogenesis, 1991, 12(12): 2297-2300.DOI:10.1093/carcin/12.12.2297.
[25] MONNIER V, SELL D, MIYATA D, et al. The maillard reaction in food processing, human nutrition and physiology[J]. Carbohydrate Polymers, 1990, 16(4): 460-461. DOI:10.1016/0144-8617(91)90064-J.
[26] HERRAIZ T, OUGH C S. Chemical and technological factors determining tetrahydro-β-carboline-3-carboxylic acid content in fermented alcoholic beverages[J]. Journal of Agricultural and Food Chemistry, 1993, 41(6): 959-964. DOI: 10.1021/jf00030a024.
[27] HERRAIZ T. Analysis of the bioactive alkaloids tetrahydro-βcarboline and β-carboline in food[J]. Journal of Chromatography A,2000, 881(1): 483-499. DOI:10.1016/S0021-9673(99)01313-8.
[28] KIKUGAWA K, HIRAMOTO K, KATO T. Prevention of the formation of mutagenic and/or carcinogenic heterocyclic amines by food factors[J]. Biofactors, 2000, 12(1/4): 123-127. DOI:10.1002/biof.5520120119.
[29] TOTSUKA Y, USHIYAMA H, ISHIHARA J, et al. Quantif i cation of the co-mutagenic beta-carbolines, norharman and harman, in cigarette smoke condensates and cooked foods[J]. Cancer Letters, 1999, 143(2):139-143. DOI:10.1016/s0304-3835(99)00143-3.
[30] PERSSON E, SJOHOLM I, NYMAN M, et al. Addition of various carbohydrates to beef burgers affects the formation of heterocyclic amines during frying[J]. Journal of Agricultural and Food Chemistry,2004, 52(25): 7561-7566. DOI:10.1021/jf0493831.
[31] 陈静. 烤牛肉饼中不同结合状态杂环胺的生成、抑制及体外消化研究[D]. 无锡: 江南大学, 2018: 1-3.
[32] ARKADIUSZ S. Chemical state of heterocyclic aromatic amines in grilled beef: evaluation by in vitro digestion model and comparison of alkaline hydrolysis and organic solvent for extraction[J]. Food and Chemical Toxicology, 2013, 62: 653-660. DOI:10.1016/j.fct.2013.09.036.
[33] KATAOKA H, MIYAKE M, NISHIOKA S, et al. Formation of protein adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in cooked foods[J]. Molecular Nutrition and Food Research, 2010,54(7): 1039-1048. DOI:10.1002/mnfr.200900066.
[34] KATAOKA H, MIYAKE M, SAITO K, et al. Formation of heterocyclic amine-amino acid adducts by heating in a model system[J]. Food Chemistry, 2012, 130(3): 725-729. DOI:10.1016/j.foodchem.2011.07.094.
[35] BORDAS M, MOYANO E, PUIGNOU L, et al. Formation and stability of heterocyclic amines in a meat fl avour model system: effect of temperature, time and precursors[J]. Journal of Chromatography B,2004, 802(1): 11-17. DOI:10.1016/j.jchromb.2003.09.024.
[36] KNIZE M G, DOLBEARE F A, CARROLL K L, et al. Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties[J]. Food and Chemical Toxicology, 1994, 32(7):595-603. DOI:10.1016/0278-6915(94)90002-7.
[37] ARVIDSSON P, VANBOEKEL M, SKOG K, et al. Kinetics of formation of polar heterocyclic amines in a meat model system[J].Journal of Food Science, 1997, 62(5): 911-916. DOI:10.1111/j.1365-2621.1997.tb15005.x.
[38] JOSHI A D, KIM A, LEWINGER J P, et al. Meat intake, cooking methods, dietary carcinogens, and colorectal cancer risk: fi ndings from the colorectal cancer family registry[J]. Cancer Medicine, 2015, 4(3):936-952. DOI:10.1002/cam4.461.
[39] KONDJOYAN A, OILLIC S, PORTANGUEN S, et al. Combined heat transfer and kinetic models to predict cooking loss during heat treatment of beef meat[J]. Meat Science, 2013, 95(2): 336-344.DOI:10.1016/j.meatsci.2013.04.061.
[40] 李利洁. 微波加热中杂环胺PhIP的形成规律及抑制机制[D]. 无锡:江南大学, 2018: 22-36.
[41] HASKARACA G, DEMIROK E, KOLSARICI N, et al. Effect of green tea extract and microwave pre-cooking on the formation of heterocyclic aromatic amines in fried chicken meat products[J]. Food Research International, 2014, 63: 373-381. DOI:10.1016/j.foodres.2014.04.001.
[42] OZ F, KABAN G, KAYA M. Effects of cooking methods and levels on formation of heterocyclic aromatic amines in chicken and fi sh with oasis extraction method[J]. LWT-Food Science and Technology, 2010,43(9): 1345-1350. DOI:10.1016/j.lwt.2010.04.014.
[43] ZORRILLA S E, SINGH R P. Heat transfer in double-sided cooking of meat patties considering two-dimensional geometry and radial shrinkage[J]. Journal of Food Engineering, 2003, 57(1): 57-65.DOI:10.1016/S0260-8774(02)00273-X.
[44] ALAEJOS M S, AFONSO A M. Factors that affect the content of heterocyclic aromatic amines in foods[J]. Comprehensive Reviews in Food Science and Food Safety, 2011, 10(2): 52-108. DOI:10.1111/j.1541-4337.2010.00141.x.
[45] ABDULKARIM B G, SMITH J S. Heterocyclic amines in fresh and processed meat products[J]. Journal of Agricultural and Food Chemistry, 1998, 46(11): 4680-4687. DOI:10.1021/jf980175g.
[46] HASNOL N D S, JINAP S, SANNY M. Effect of different types of sugars in a marinating formulation on the formation of heterocyclic amines in grilled chicken[J]. Food Chemistry, 2014, 145: 514-521.DOI:10.1016/j.foodchem.2013.08.086.
[47] SKOG K, JAGERSTAD M. Effects of monosaccharides and disaccharides on the formation of food mutagens in model systems[J].Mutation Research, 1990, 230(2): 263-272. DOI:10.1016/0027-5107(90)90064-B.
[48] YU Shujuan, YU Di. Effects of some cations on the formation of 2-amino-1-methyl-6- phenylimidazo[4,5-b]pyridine (PhIP) in a model system[J]. Food Chemistry, 2016, 201: 46-51. DOI:10.1016/j.foodchem.2016.01.066.
[49] SKOG K, SOLYAKOV A, JÄGERSTAD M. Effects of heating conditions and additives on the formation of heterocyclic amines with reference to aminocarbolines in a meat juice model system[J]. Food Chemistry, 2000, 68(3): 299-308. DOI:10.1016/S0308-8146(99)00195-8.
[50] MAÏA M, ERWAN E. Mitigation strategies to reduce the impact of heterocyclic aromatic amines in proteinaceous foods[J]. Trends in Food Science and Technology, 2016, 50: 70-84. DOI:10.1016/j.tifs.2016.01.007.
[51] SEPAHPOUR S, SELAMAT J, KHATIB A, et al. Inhibitory effect of mixture herbs/spices on formation of heterocyclic amines and mutagenic activity of grilled beef[J]. Food Additives and Contaminants Part A: Chemistry, Analysis, Control, Exposure and Risk Assessment,2018, 35(10): 1911-1927. DOI:10.1080/19440049.2018.1488085.
[52] SHAO Zeping, HAN Zhonghui, ZHANG Jinhui, et al. Inhibition effects of flavonoids on 2-amino-3,8-dimethyl-imidazo[4,5-f]-quinoxaline and 2-amino-3,7,8-trimethyl-imidazo[4,5-f]-quinoxaline formation and alkoxy radical scavenging capabilities of fl avonoids in a model system[J]. Journal of the Science of Food and Agriculture,2018, 98(8): 2908-2914. DOI:10.1002/jsfa.8785.
[53] KESKEKOGLU H, UREN A. Inhibitory effects of grape seed extract on the formation of heterocyclic aromatic amines in beef and chicken meatballs cooked by different techniques[J]. International Journal of Food Properties, 2017, 20(Suppl 1): S722-S734. DOI:10.1080/109429 12.2017.1308956.
[54] ZENG Maomao, ZHANG Mengru, ZHANG Shuang, et al. Inhibitory prof i les of chilli pepper and capsaicin on heterocyclic amine formation in roast beef patties[J]. Food Chemistry, 2017, 221: 404-411.DOI:10.1016/j.foodchem.2016.10.061.
[55] SABALLY K, SLENO L, JAUFFRIT J A, et al. Inhibitory effects of apple peel polyphenol extract on the formation of heterocyclic amines in pan fried beef patties[J]. Meat Science, 2016, 117: 57-62.DOI:10.1016/j.meatsci.2016.02.040.
[56] JAMALI M A, ZHANG Y, TENG H, et al. Inhibitory effect of Rosa rugosa tea extract on the formation of heterocyclic amines in meat patties at different temperatures[J]. Molecules, 2016, 21(2): 173-186.DOI:10.3390/molecules21020173.
[57] ROUNDS L, HAVENS C M, FEINSTEIN Y, et al. Plant extracts,spices, and essential oils inactivate Escherichia coli O157:H7 and reduce formation of potentially carcinogenic heterocyclic amines in cooked beef patties[J]. Journal of Agricultural and Food Chemistry,2012, 60(14): 3792-3799. DOI:10.1021/jf204062p.
[58] YU Shujuan, YU Di, CHEN Mingshun. Effect of sugarcane molasses extract on the formation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in a model system[J]. Food Chemistry, 2016,197(PtA): 924-929. DOI:10.1016/j.foodchem.2015.11.052.
[59] KHAN I A, LIU D, YAO M, et al. Inhibitory effect of Chrysanthemum morifolium flower extract on the formation of heterocyclic amines in goat meat patties cooked by various cooking methods and temperatures[J]. Meat Science, 2019, 147: 70-81. DOI:10.1016/j.meatsci.2018.08.028.
[60] JIAO Ye, YAN Yan, HE Zhiyong, et al. Inhibitory effects of catechins on β-carbolines in tea leaves and chemical model systems[J]. Food and Function, 2018, 9(6): 3126-3133. DOI:10.1039/c7fo02053h.
[61] 姚瑶. 酱牛肉中常见杂环胺化合物的形成与控制研究[D]. 南京: 南京农业大学, 2013: 71-86.
[62] KIKUGAWA K, HIRAMOTO K, KATO T, et al. Effect of food reductones on the generation of the pyrazine cation radical and on the formation of the mutagens in the reaction of glucose, glycine and creatinine[J]. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2000, 465(1): 183-190. DOI:10.1016/S1383-5718(99)00227-2.
[63] KIKUGAWA K, KATO T, HIRAMOTO K, et al. Participation of the pyrazine cation radical in the formation of mutagens in the reaction of glucose/glycine/creatinine[J]. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 1999, 444(1): 133-144. DOI:10.1016/S1383-5718(99)00086-8.
[64] VIEGAS O, MOREIRA P S, FERREIRA I M. Influence of beer marinades on the reduction of carcinogenic heterocyclic aromatic amines in charcoal-grilled pork meat[J]. Food Additives and Contaminants-Part A: Chemistry, Analysis, Control, Exposure and Risk Assessment, 2015, 32(3): 315-323. DOI:10.1080/19440049.2015.1010607.
[65] MERCAN M T, MEVLUDE K. Reducing effect of artichoke extract on heterocyclic aromatic amine formation in beef and chicken breast meat[J]. Meat Science, 2017, 134: 68-75. DOI:10.1016/j.meatsci.2017.07.018.
[66] 秦川. 膳食类黄酮抑制烤牛肉饼中杂环胺PhIP的形成作用研究[D].无锡: 江南大学, 2014: 13-31.
[67] ZHOU Bin, ZHAO Yueliang, WANG Xichang, et al. Unraveling the inhibitory effect of dihydromyricetin on heterocyclic aromatic amines formation[J]. Journal of the Science of Food and Agriculture, 2018,98(5): 1988-1994. DOI:10.1002/jsfa.8682.
[68] WONG D, CHENG K W, WANG M. Inhibition of heterocyclic amine formation by water-soluble vitamins in Maillard reaction model systems and beef patties[J]. Food Chemistry, 2012, 133(3): 760-766.DOI:10.1016/j.foodchem.2012.01.089.
[69] LINGHU Z Y, KARIM F, SMITH J S. Amino acids inhibitory effects and mechanism on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine(PhIP) formation in the Maillard reaction model systems[J]. Journal of Food Science, 2017, 82(12): 3037-3045. DOI:10.1111/1750-3841.13959.
[70] 张梦茹. 辛辣味香辛料及其特征成分对烤牛肉饼中杂环胺生成规律的影响研究[D]. 无锡: 江南大学, 2017: 17-41.
[71] ZENG Maomao, WANG Junhui, ZHANG Mengru, et al. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties[J]. Food Chemistry, 2018, 239: 111-118. DOI:10.1016/j.foodchem.2017.06.097.
[72] SHIN H S. Inhibition of heterocyclic aromatic amine formation in fried ground beef patties by organosulfur compounds in garlic[D]. East Lansing: Michigan State University, 2001: 51.
[73] JANOSZKA B. Heterocyclic amines and azaarenes in pan-fried meat and its gravy fried without additives and in the presence of onion and garlic[J]. Food Chemistry, 2010, 120(2): 463-473. DOI:10.1016/j.foodchem.2009.10.039.
[74] JUNG K, LEE K, PARK J, et al. Inf l uence of fructooligosaccharides and garlic on formation of heterocyclic amines in fried ground beef patties[J]. Food Science and Biotechnology, 2010, 19(5): 1159-1164.DOI:10.1007/s10068-010-0165-0.
[75] TSAI S J, JENQ S N, LEE H. Naturally occurring diallyl disulfide inhibits the formation of carcinogenic heterocyclic aromatic amines in boiled pork juice[J]. Mutagenesis, 1996, 11(3): 235-240. DOI:10.1093/mutage/11.3.235.
[76] SHIN H S, STRASBURG G M, GRAY J I. A model system study of the inhibition of heterocyclic aromatic amine formation by organosulfur compounds[J]. Journal of Agricultural and Food Chemistry, 2002, 50: 7684-7690. DOI:10.1021/jf05707e.
[77] PUANGSOMBAT K, JIRAPAKKUL W, SMITH J S, et al. Inhibitory activity of asian spices on heterocyclic amines formation in cooked beef patties[J]. Journal of Food Science, 2011, 76(8): 174-180.DOI:10.1111/j.1750-3841.2011.02338.x.
[78] SHIN H S, RODGERS W J, STRASBURG G M, et al. Reduction of heterocyclic aromatic amine formation and overall mutagenicity in fried ground beef patties by organosulfur compounds[J]. Journal of Food Science, 2002, 67: 3304-3308. DOI:10.1111/j.1365-2621.2002.tb09583.x.
[79] GIBIS M, WEISS J. Inhibitory effect of cellulose fibers on the formation of heterocyclic aromatic amines in grilled beef patties[J]. Food Chemistry, 2017, 229: 828-836. DOI:10.1016/j.foodchem.2017.02.130.
[80] PERSSON E, SJOHOLM I, SKOG K. Effect of high waterholding capacity on the formation of heterocyclic amines in fried beefburgers[J]. Journal of Agricultural and Food Chemistry, 2003,51(15): 4472-4477. DOI:10.1021/jf021089q.
[81] MONTI S M, RITIENI A, SACCHI R, et al. Characterization of phenolic compounds in virgin olive oil and their effect on the formation of carcinogenic/mutagenic heterocyclic amines in a model system[J]. Journal of Agricultural and Food Chemistry, 2001, 49(8):3969-3975. DOI:10.1021/jf010240d.
[82] JAUTZ U, GIBIS M, MORLOCK G E. Quantif i cation of heterocyclic aromatic amines in fried meat by HPTLC/UV-FLD and HPLC/UVFLD: a comparison of two methods[J]. Journal of Agricultural and Food Chemistry, 2008, 56(12): 4311-4319. DOI:10.1021/jf800689h.
Recent Progress in the Formation and Inhibition of Heterocyclic Aromatic Amines in Cooked Meat Products
ZHANG Yuxia, ZHOU Yajun*, LI Shengrao
(College of Food Science and Engineering, Jilin University, Changchun 130062, China)
Abstract: Heterocyclic aromatic amines (HAAs) are the most common chemical hazards in cooked meat products. A wide variety of HAAs have been detected in cooked meat products, which are formed through complicated pathways. Various mechanisms/methods have been developed to inhibit the formation of HAAs during the processing of cooked meat products.This paper describes the structure of two classes of HAAs, with a focus on the formation pathways of quinoline, quinoxaline,2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), β-carboline (1-methyl-9H-pyrido[4,3-b]indole (harman),9H-pyrido[4,3-b]indole (norharman)) and bound HAAs. It also summarizes the measures and mechanisms to reduce the formation of HAAs (controlling processing conditions and precursors, adding foreign substances to eliminate free radicals and intermediates, inhibiting the Maillard reaction), and brief l y reviews the present status of research on HAAs.
Keywords: heterocyclic aromatic amine; cooked meat products; formation; inhibition; mechanism
收稿日期:2019-05-07
基金项目:“十三五”国家重点研发计划重点专项(2018YFD0401200;2016YFD0401500)
第一作者简介:张玉霞(1994—)(ORCID: 0000-0001-8618-8072),女,博士研究生,研究方向为肉品科学与加工新技术。E-mail: ssrszhangyx@163.com
*通信作者简介:周亚军(1966—)(ORCID: 0000-0003-4541-3389),男,教授,博士,研究方向为肉品科学与加工新技术。E-mail: zhouruyilang@163.com
DOI:10.7506/rlyj1001-8123-20190507-099
中图分类号:TS251.6
文献标志码:A
文章编号:1001-8123(2019)08-0065-09
引文格式:张玉霞, 周亚军, 李圣桡. 熟肉制品中杂环胺的形成与抑制研究进展[J]. 肉类研究, 2019, 33(8): 65-73. DOI:10.7506/rlyj1001-8123-20190507-099. http://www.rlyj.net.cn
ZHANG Yuxia, ZHOU Yajun, LI Shengrao. Recent progress in the formation and inhibition of heterocyclic aromatic amines in cooked meat products[J]. Meat Research, 2019, 33(8): 65-73. DOI:10.7506/rlyj1001-8123-20190507-099. http://www.rlyj.net.cn





