表1 不同TG酶添加量鸭胸肉糜的配方
Table 1 Formulation of duck breast meat batters with various amounts of TG
g
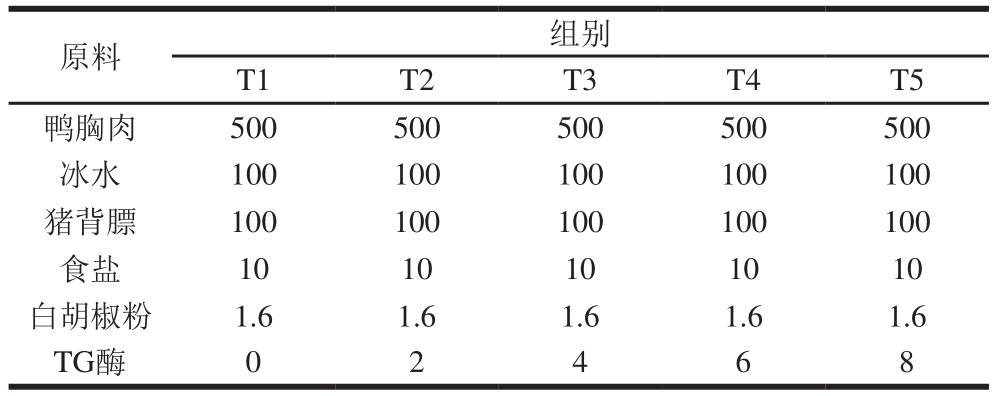
原料 组别T1 T2 T3 T4 T5鸭胸肉 500 500 500 500 500冰水 100 100 100 100 100猪背膘 100 100 100 100 100食盐 10 10 10 10 10白胡椒粉 1.6 1.6 1.6 1.6 1.6 TG酶 0 2 4 6 8
随着肉鸭养殖业技术的成熟和规模的扩大,我国鸭肉产量逐年增加,已成为世界上最大的鸭肉生产和消费国[1]。鸭肉营养丰富,性价比高[2],在重组肉制品,如肉肠、肉丸等中大量使用[3-4]。肌原纤维蛋白是鸭肉中的主要蛋白质,也是生鲜鸭肉糜液体连续相中的主要组成部分。加工过程中通过添加食盐和磷酸盐类物质将肌原纤维蛋白提取出来,包裹在脂肪液滴或颗粒周围,形成稳定的乳化肉糜体系,这决定着肉糜凝胶的保水性和质构。但加工不当易造成肌原纤维蛋白变性,引起蒸煮损失增大、结构松散等问题[5]。为了解决这个问题,工厂生产中常常添加谷氨酰胺转氨酶(transglutaminase,TG)催化蛋白质谷氨酰胺残基的γ-羧酰和赖氨酸残基的ζ-氨基发生转酰胺基反应,形成ζ—(γ—Glu)Lys共价键,或肽链中谷氨酰胺残基的羧酰胺和伯胺之间的酰胺基发生转移反应,并使蛋白质分子之间聚合,增强蛋白质分子内部、分子之间的共价交联以及氨基酸和蛋白质之间的交联,改善凝胶肉制品的品质[6-8]。但TG酶对鸭胸肉凝胶性能和水分分布状态及迁移影响的研究较少,因此,本研究针对TG酶添加量对鸭胸肉糜色泽、保水性和质构等的影响进行研究。
TG酶(含1% TG酶和99%麦芽糊精) 江苏省泰兴市东圣食品科技有限公司;冷冻樱桃谷鸭胸肉 青岛和鑫源盛国际贸易有限公司;猪背膘、白砂糖、食盐、白胡椒粉 河南新乡超市。
FA224电子天平 上海舜宇恒平科学仪器有限公司;CR-400色差计 日本柯尼卡-美能达公司;PQ00l台式核磁共振(nuclear magnetic resonance,NMR)分析仪上海纽迈电子有限公司;DZKW-4 电子恒温水浴锅北京中兴伟业仪器有限公司;AUX-J22绞肉机 佛山市海迅电器有限公司;UMC-5C斩拌机 德国Stephan公司;HH-42水浴锅 常州国华电器有限公司;TA-XT.plus质构仪 英国Stable Micro System公司;Beckman L-80-XP离心机 美国Ultracentrifuge公司;Testo 905-T1插入式温度计 徐州亚名仪器仪表有限公司。
1.3.1 鸭胸肉糜的制备
将冷冻鸭胸肉放入0~4 ℃环境中解冻至中心温度为0 ℃左右,去除鸭皮、脂肪及其他杂质,应用孔板直径6 mm的绞肉机绞碎,存放于0~4 ℃冰箱中直到加工,6 h内应用完毕。鸭胸肉糜配方如表1所示。鸭胸肉糜加工方法参考Kang Zhuangli等[9]的方法。将肉糜制成直径为30 mm的肉丸,放入80 ℃水浴锅中煮制20 min,至中心温度72 ℃(使用插入式温度计实时监控),捞出水冷至室温后放入0~4 ℃冷库中待用。
表1 不同TG酶添加量鸭胸肉糜的配方
Table 1 Formulation of duck breast meat batters with various amounts of TG
g

原料 组别T1 T2 T3 T4 T5鸭胸肉 500 500 500 500 500冰水 100 100 100 100 100猪背膘 100 100 100 100 100食盐 10 10 10 10 10白胡椒粉 1.6 1.6 1.6 1.6 1.6 TG酶 0 2 4 6 8
1.3.2 色差的测定
将冷却后的鸭肉丸取出,从中间切开,使用色差计对切面进行测定,测定时间不超过5 min。标准白色比色板的亮度值(L*)、红度值(a*)和黄度值(b*)分别为96.86、-0.15和1.87。
1.3.3 蒸煮得率的测定
冷却过夜后,分别对每份肉糜的质量进行测定,按照下式计算蒸煮得率,每组测定4 次。
1.3.4 质构的测定
将肉糜放置在20 ℃室温下回温2 h后使用P/36探头进行测定。样品修整为直径20 mm、高20 mm的圆柱体,测定参数如下:测试速率2.0 mm/s,压缩比50%,时间5 s。得到硬度、弹性、内聚性和咀嚼性[10]。每组测定6 次。
1.3.5 NMR自旋-自旋驰豫时间(T2)的测定
取2 g左右的肉糜,放入直径为15 mm的核磁管后,放入PQ001纽迈台式脉冲NMR分析仪中进行测定。参数如下:温度32 ℃,质子共振频率22.6 MHz,τ-值200 μs。重复扫描32 次,重复间隔时间6.5 s,得到12 000 个回波,每组测定4 次。
应用SPSS 18.0软件(SPSS Inc.,USA)进行数据统计,使用单因素方差分析(analysis of variance,ANOVA)对数据进行分析,P<0.05时组间存在显著差异。
由图1可知,从T1组到T4组鸭胸肉糜,蒸煮得率随着TG酶添加量的增加而显著提高(P<0.05),而T4组和T5组差异不显著(P>0.05)。Tammatinna等[11]研究TG酶对白对虾凝胶特性的影响时发现,TG酶添加量越高,肌球蛋白重链的聚集度就越高,内部和外部肽链之间的交联增强,形成良好的凝胶结构,能够提高保水性[12]。T4组和T5组鸭胸肉糜的蒸煮得率差异不显著(P>0.05),这是由于较高的TG酶添加量加快了蛋白质的交联和结合速率,减弱了蛋白质和水分之间的结合[13]。康壮丽等[14]发现,鸡肉糜的乳化稳定性和蒸煮得率随着TG酶添加量的增加而显著提高(P<0.05),但0.67%和1.00%的添加量间差异不显著(P>0.05)。

图1 TG酶添加量对鸭胸肉糜蒸煮得率的影响
Fig. 1 Effect of TG on cooking yield of duck breast meat batter
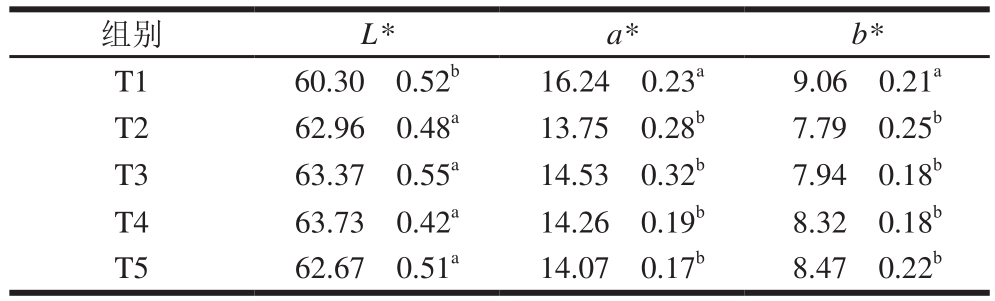
表2 TG酶添加量对鸭胸肉糜色差的影响
Table 2 Effect of TG on color of duck breast meat batter
注:同列小写字母不同,表示差异显著(P<0.05)。表3~5同。
组别 L* a* b*T1 60.30f0.52b 16.24f0.23a 9.06f0.21a T2 62.96f0.48a 13.75f0.28b 7.79f0.25b T3 63.37f0.55a 14.53f0.32b 7.94f0.18b T4 63.73f0.42a 14.26f0.19b 8.32f0.18b T5 62.67f0.51a 14.07f0.17b 8.47f0.22b
由表2可知,添加TG酶显著提高了鸭胸肉糜的L*,降低了a*和b*(P<0.05),而TG酶的添加量对L*、a*和b*影响不显著(P>0.05)。添加TG酶增强了蛋白质之间的交联,提高了蒸煮鸭胸肉糜的水分含量,增强了肉糜表面对光线的反射,造成鸭胸肉糜a*和b*显著降低(P<0.05),L*显著升高(P<0.05)。Kilic[15]发现,添加TG酶显著提高鸡肉的L*(P<0.05),而TG酶添加量对L*影响不显著(P>0.05)。
表3 TG酶添加量对鸭胸肉糜质构的影响
Table 3 Effect of TG on texture of duck breast meat batter
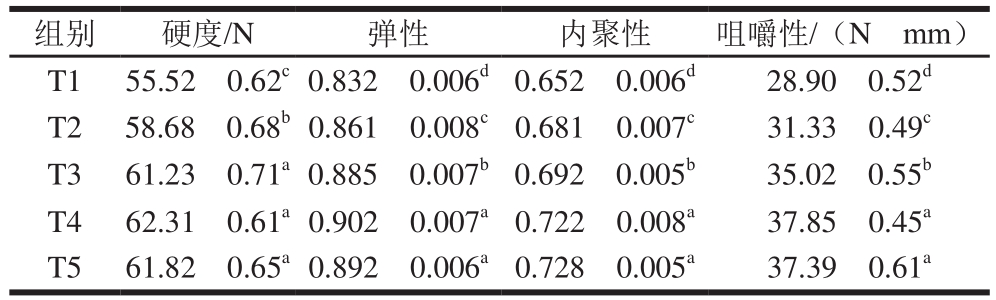
组别 硬度/N 弹性 内聚性 咀嚼性/(Ngmm)T1 55.52f0.62c0.832f0.006d0.652f0.006d 28.90f0.52d T2 58.68f0.68b0.861f0.008c0.681f0.007c 31.33f0.49c T3 61.23f0.71a0.885f0.007b0.692f0.005b 35.02f0.55b T4 62.31f0.61a0.902f0.007a0.722f0.008a 37.85f0.45a T5 61.82f0.65a0.892f0.006a0.728f0.005a 37.39f0.61a
由表3可知:TG酶添加量增加时,鸭胸肉糜的硬度从(55.52f0.62) N提高到(61.82f0.65) N,但T3、T4和T5组的硬度差异不显著(P>0.05);鸭胸肉糜的弹性、内聚性和咀嚼性随着TG酶添加量的增加而显著增大(P<0.05),但T4和T5组差异不显著(P>0.05)。TG酶诱导肌肉蛋白质发生交联和聚集,有利于凝胶结构的形成。在蛋白含量(底物)不变的情况下,TG酶超过最佳添加量不能够再改善鸭胸肉糜的质构特性。这与Herrero等[16]的研究结果一致,他们发现,添加TG酶能显著改善肉糜体系的结构和质构特性。Carballo等[17]研究发现,添加TG酶和酪蛋白酸钠提高了蒸煮肉糜的硬度和咀嚼性。Kang Zhuangli等[18]也发现,TG酶添加量从0%提高到1%能显著提高法兰克福香肠的硬度、弹性、内聚性和咀嚼性,这是由于添加TG酶诱导蛋白质二级结构发生变化,β-转角和β-折叠含量显著增加,而α-螺旋和无规则卷曲含量显著降低(P>0.05),其中β-折叠结构是凝胶形成的基础[19-20]。
表4 TG酶添加量对鸭胸肉糜弛豫时间的影响
Table 4 Effect of TG on relaxation time of duck breast meat batter ms

组别 T2b T21 T22 T1 0.73f0.04a 65.62f1.55a 284.41f4.27a T2 0.56f0.05b 54.35f1.65b 265.25f4.38b T3 0.52f0.04b 51.03f1.52c 238.68f4.57c T4 0.58f0.06b 46.65f1.61d 211.74f4.16d T5 0.54f0.05b 45.77f1.58d 206.32f4.52d
肉糜体系中水分的分布能用质子自旋-自旋弛豫时间(T2)来反映[21-22]。由表4可知,共出现3 个特征峰:T2b、T21和T22。大量研究发现,起始弛豫时间0~10、20~100、250~400 ms分别表示结合水(T2b)、可移动水(T21)和游离水(T22)[23-26]。与T1组相比,添加TG酶能够显著缩短T2b、T21和T22(P<0.05)。起始弛豫时间T2越短,说明水分子与底物结合越紧密,反之结合越松散[27-29],表明添加TG酶使蒸煮鸭胸肉糜中的水分子与底物结合更紧密。提高TG酶添加量,T21和T22起始弛豫时间显著缩短(P<0.05),表明可移动水和游离水分子移动性降低;而T2、T3、T4和T5组的T2b起始弛豫时间差异不显著(P>0.05),T4和T5组的T21和T22起始弛豫时间差异不显著(P>0.05),表明在一定添加量范围内TG酶对结合水、可移动水和游离水的影响较小。
表5 TG酶添加量对鸭胸肉糜峰面积比例的影响
Table 5 Effect of TG on ratio of relaxation peak areas in duck breast meat batter
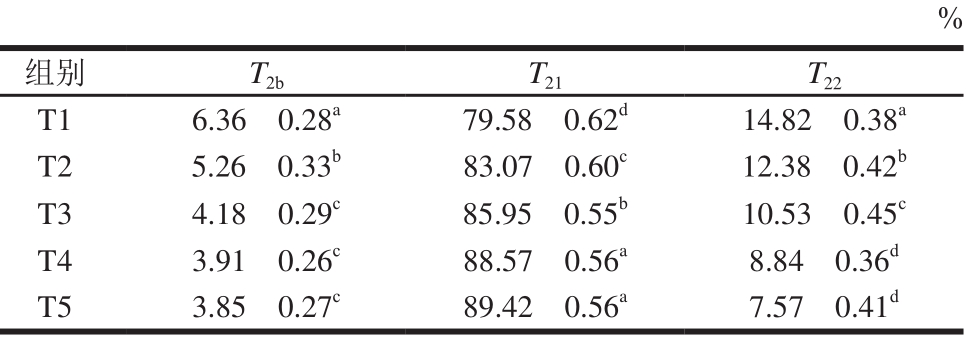
%组别 T2b T21 T22 T1 6.36f0.28a 79.58f0.62d 14.82f0.38a T2 5.26f0.33b 83.07f0.60c 12.38f0.42b T3 4.18f0.29c 85.95f0.55b 10.53f0.45c T4 3.91f0.26c 88.57f0.56a 8.84f0.36d T5 3.85f0.27c 89.42f0.56a 7.57f0.41d
由表5可知,不同TG酶添加量蒸煮鸭胸肉糜中不同状态水的峰面积比例差异显著(P<0.05)。添加TG酶后,T2b的峰面积比例降低,主要原因是鸭胸肉糜的保水性增强(图1),不易流动水的比例降低;提高TG酶添加量后,可移动水(T21)的峰面积比例增加,游离水(T22)的峰面积比例降低,但T4和T5组的T21和T22峰面积比例差异不显著(P>0.05),说明在一定范围内提高TG酶添加量有利于鸭胸肉糜凝胶结构的形成。将水分束缚于鸭胸肉糜中,能够减少蒸煮过程中水分的损失[30-31]。NMR弛豫结果表明,在一定范围内提高TG酶添加量能够提高鸭胸肉糜的保水性。
添加TG酶对鸭胸肉凝胶性能的影响显著。与不添加TG酶的鸭胸肉糜相比,添加TG酶显著提高了鸭胸肉糜的L*、蒸煮得率、硬度、弹性、内聚性和咀嚼性,降低了a*和b*(P<0.05),过量添加TG酶对鸭胸肉糜的色泽、保水性和质构影响不显著(P>0.05)。添加TG酶显著缩短了鸭胸肉糜的T2b、T21和T22起始弛豫时间(P<0.05),降低了T2b和T22的峰面积比例,提高了T21的峰面积比例,表明水分的流动性降低。
[1] 韩吉娜, HIPPOLYTE K S, 杨鸿博, 等. 气调包装对冷却鸭肉的保鲜效果研究[J]. 食品与发酵工业, 2019, 45(9): 159-164. DOI:10.13995/j.cnki.11-1802/ts.018894.
[2] 马汉军, 周光宏, 余小领, 等. 鸭肉肠的加工工艺研究[J]. 食品科学,2008, 29(11): 183-185.
[3] 李超, 耿中华, 商学兵, 等. 气调包装保鲜冷却鸭肉的研究[J]. 食品工业, 2012(1): 115-117.
[4] SIKES A L, TOBIN A B, TUME R K. Use of high pressure to reduce cook loss and improve texture of low-salt beef sausage batters[J].Innovative Food Science and Emerging Technologies, 2009, 10:405-412. DOI:10.1016/j.ifset.2009.02.007.
[5] VERMA A K, BANERJEE R. Low-sodium meat products: retaining salty taste for sweet health[J]. Critical Review Food Science Nutrition,2012, 52(1): 72-84. DOI:10.1080/10408398.2010.498064.
[6] CARDOSO C, MENDES R, VAZPIRES P, et al. Effect of salt and MTGase on the production of high quality gels from farmed sea bass[J]. Journal of Food Engineering, 2010, 101(1): 98-105.DOI:10.1016/j.jfoodeng.2010.06.017.
[7] LU X, HRYNETS Y, BETTI M, et al. Transglutaminase-catalyzed amination of pea protein peptides using the biogenic amines histamine and tyramine[J]. Journal of the Science of Food and Agriculture, 2017,97(8): 2436-2442. DOI:10.1002/jsfa.8057.
[8] TOKAY F G, YERLIKAYA P. Shelf-life extension of fish fillets by spraying with microbial transglutaminase[J]. Journal of Aquatic Food Product Technology, 2017, 26(8): 940-948. DOI:10.1080/10498850.20 17.1363338.
[9] KANG Zhuangli, LI Ke, MA Hanjun, et al. Effect of different processing methods and salt content on the physicochemical and rheological properties of meat batters[J]. International Journal of Food Properties,2016, 19(7): 1604-1615. DOI:10.1080/10942912.2015.1105819.
[10] BOURNE M C. Texture profile analysis[J]. Food Technology, 1978,32: 62-66.
[11] TAMMATINNA A, BENJAKUL S, VISESSANGUAN W, et al.Gelling properties of white shrimp (Penaeus vannamei) meat as influenced by setting condition and microbial transglutaminase[J].LWT-Food Science and Technology, 2007, 40(9): 1489-1497.DOI:10.1016/j.lwt.2006.11.017.
[12] GASPAR A L C, GOES-FAVONI S P. Action of microbial transglutaminase (MTGase) in the modification of food proteins:a review[J]. Food Chemistry, 2015, 171: 315-322. DOI:10.1016/j.foodchem.2014.09.019.
[13] AHHMED A M, NASU T, MUGURUMA M. Impact of transglutaminase on the textural, physicochemical, and structural properties of chicken skeletal, smooth, and cardiac muscles[J]. Meat Science, 2009, 83: 759-767. DOI:10.1016/j.meatsci.2009.08.018.
[14] 康壮丽, 李想, 李斌, 等. TG酶对低盐鸡肉糜保水性和蛋白质二级结构的影响[J]. 食品工业科技, 2016, 37(20): 130-133.
[15] KILIC B. Effect of microbial transglutaminase and sodium caseinate on quality of chicken dӧner kebab[J]. Meat Science, 2003, 63(3):417-421. DOI:10.1016/s0309-1740(02)00102-x.
[16] HERRERO A M, CAMBERO M I, ORDONEZ J A. et al.Raman spectroscopy study of the structural effect of microbial transglutaminase on meat systems and its relationship with textural characteristics[J]. Food Chemistry, 2008, 109: 25-32. DOI:10.1016/j.foodchem.2007.12.003.
[17] CARBALLO J L, AYO J, COLMENERO F J, et al. Microbial transglutaminase and caseinate as cold set binders: influence of meat species and chilling storage[J]. LWT-Food Science and Technology,2006, 39(6): 692-699. DOI:10.1016/j.lwt.2005.03.020.
[18] KANG Zhuangli, WANG Peng, XU Xinglian, et al. Effect of beating processing, as a means of reducing salt content in frankfurters: a physico-chemical and Raman spectroscopic study[J]. Meat Science,2014, 98(2): 171-177. DOI:10.1016/j.meatsci.2014.05.025.
[19] KANG Zhuangli, LI Xiang, MA Hanjun, et al. Effect of the levels of transglutaminase in frankfurters: a physical-chemical and Raman spectroscopy study[J]. CYTA-Journal of Food, 2016, 15(1): 75-80.DOI:10.1080/19476337.2016.1214928.
[20] CHEN Hongye, HAN Minyi. Raman spectroscopic study of the effects of microbial transglutaminase on heat-induced gelation of pork myofibrillar proteins and its relationship with textural characteristics[J]. Food Research International, 2011, 44(5): 1514-1520. DOI:10.1016/j.foodres.2011.03.052.
[21] LI Chunbao, LIU Dengyong, ZHOU Guanghong, et al. Meat quality and cooking attributes of thawed pork with different low field NMR T21[J]. Meat Science, 2012, 92: 79-83. DOI:10.1016/j.meatsci.2011.11.015.
[22] SHAO Junhua, DENG Yamin, JIA Na, et al. Low-field NMR determination of water distribution in meat batters with NaCl and polyphosphate addition[J]. Food Chemistry, 2016, 44(5): 308-314.DOI:10.1016/j.foodchem.2016.01.013.
[23] GOETZ J, KOEHLER P. Study of the thermal denaturation of selected proteins of whey and egg by low resolution NMR[J]. LWTFood Science and Technology, 2005, 38(5): 501-512. DOI:10.1016/j.lwt.2004.07.009.
[24] BERTRAM H C, ANDERSEN H J, KARLSSON A H. Comparative study of low-field NMR relaxation measurements and two traditional methods in the determination of water holding capacity of pork[J].Meat Science, 2001, 57(2): 125-132. DOI:10.1016/S0309-1740(00)00080-2.
[25] 韩敏义. 费英. 徐幸莲. 等. 低场NMR研究pH对肌原纤维蛋白热诱导凝胶的影响[J]. 中国农业科学, 2009, 42(6): 2098-2104.DOI:10.3864/j.issn.0578-1752.2009.06.028.
[26] TORNBERG E, WAHLGREN M, BRØNDUM J, et al. Pre-rigor conditions in beef under varying temperature and pH falls studied with rigometer, NMR and NIR[J]. Food Chemical, 2000, 69(4): 407-418.DOI:10.1016/S0308-8146(00)00053-4.
[27] BROWN R J S, CAPOZZI F, CAVANI C, et al. Relationships between1H NMR relaxation data and some technological parameters of meat:a chemometric approach[J]. Journal of Magnetic Resonance, 2000,147(1): 89-94. DOI:10.1006/jmre.2000.2163.
[28] 王梦娇, 杨菊梅, 王松磊, 等. 基于低场核磁共振技术冷鲜羊肉品质快速无损检测研究[J]. 宁夏工程技术, 2017, 16(1): 10-14.
[29] AWAD T S, MOHARRAM H A, SHALTOUT O E, et al.Applications of ultrasound in analysis, processing and quality control of food: a review[J]. Food Research International, 2012, 48(2):410-427. DOI:10.1016/j.foodres.2012.05.004.
[30] 甄少波, 刘奕忍, 郭慧媛, 等. 低场核磁共振分析猪肉宰后成熟过程中的水分变化[J]. 食品工业科技, 2017, 38(22): 66-70.
[31] LI Ke, KANG Zhuangli, ZHAO Yingying, et al. Use of high-intensity ultrasound to improve functional properties of batter suspensions prepared from PSE-like chicken breast meat[J]. Food and Bioprocess Technology, 2014, 7(12): 3466-3477. DOI:10.1007/s11947-014-1358-y.
Impact of Transglutaminase on Gel Properties of Duck Breast Meat Batter
MENG Lin, LI Yanping, KANG Zhuangli, et al. Impact of transglutaminase on gel properties of duck breast meat batter[J].Meat Research, 2019, 33(8): 25-28. DOI:10.7506/rlyj1001-8123-20190617-131. http://www.rlyj.net.cn